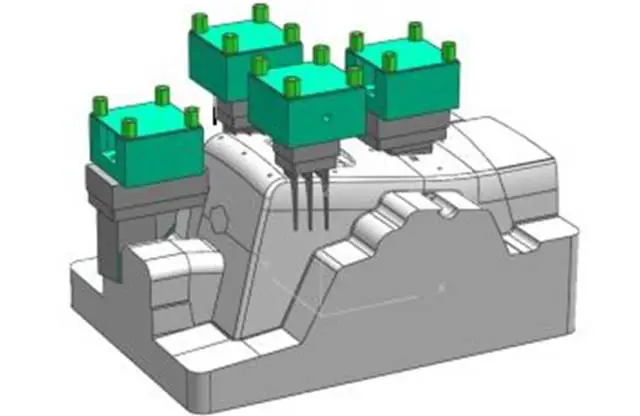
With advancements in science, technology, and innovations in mechanical part processing, society now demands higher precision in medical device deburring and polishing, stricter sterilization standards for surfaces, and improved compatibility of implant materials with the human body.
In response, leading medical device manufacturers are pursuing more reliable and efficient surface finishing technologies to enhance the quality and added value of orthopedic products. This article introduces four key surface processing technologies for orthopedic instruments and implants.
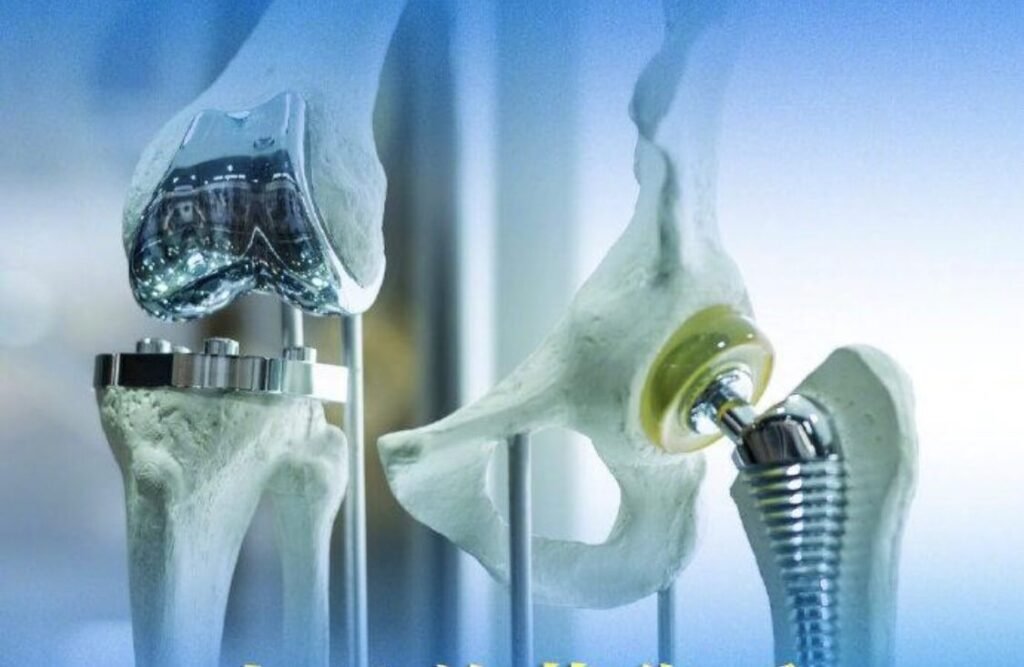
1. Precision Grinding Technology
Orthopedic implants mainly include bone fixation and joint implants, such as spinal fixation devices and artificial joints (e.g., hip and knee implants). Depending on clinical needs, the roundness, size, and surface roughness of artificial hip joints are critical quality indicators.
According to data from Australia, the cumulative 5-year revision rate after hip surface replacement surgery has increased significantly. To improve fatigue resistance, surface treatment processes such as grinding, honing, and final polishing are necessary to achieve excellent surface quality and dimensional accuracy.
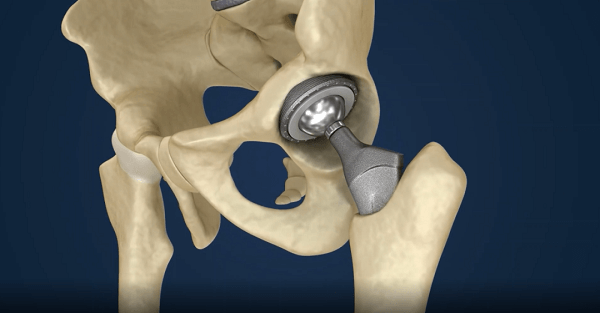
Precision grinding uses a grinding wheel to machine raw materials to specific tolerances. This not only minimizes error but also prepares the surface for subsequent honing, which can achieve mirror-like smoothness and uniform geometric tolerances.
2. Deburring and Polishing Technology
Implants must be completely free of burrs or defects, as even microscopic surface irregularities can increase the risk of infection and slow down the process of biological integration with body tissues.
Polishing, as the final finishing process, is especially crucial in hip replacement surgeries. Its purpose is to reduce surface roughness, lower friction and wear, and eliminate burrs, tool marks, and machining traces.
Modern techniques allow for burr-free and microcrack-free finishing without altering the metal’s internal structure. For instance, high-precision electrochemical machining (PECM) enables non-contact metal removal, achieving surface roughness up to Ra 0.03 μm. This process produces burr-free components with submicron-level dimensional accuracy and repeatability, resulting in a truly mirror-like finish.
3. Cleaning and Sterilization Technology
The cleaning process must meet two strict criteria:
- Ensuring the cleanliness and sterility of surgical instruments to prevent infection and complications.
- Prolonging the service life of medical devices, especially those frequently used in orthopedics, such as bone prostheses, artificial joints, and fixation plates.

Common cleaning methods include manual and automated processes, such as automatic washers and ultrasonic cleaning. For porous or structurally complex instruments where conventional washing is ineffective, ultrasonic cleaning provides superior results.
4. Surface Coating Technology
Taking total hip arthroplasty as an example, aseptic loosening is the primary cause requiring revision surgery. This necessitates enhancing the compatibility of implant materials to enable long-term coexistence with bone tissue. This involves coating technologies that promote osseointegration and provide antimicrobial properties to improve the performance and service life of bone-joint prostheses.
Coating technologies aimed at promoting osseointegration utilize specific biomolecular coatings based on biofunctional principles. These enable bone cells to directly adhere to biocompatible materials, providing an ideal material interface that participates in the bone repair process. Coatings primarily focused on antimicrobial properties achieve bactericidal effects by inhibiting bacterial growth while maintaining the specialized microstructure required for bone surfaces.
Common coatings include the osteo-mimetic material hydroxyapatite (HA) and the metallic material tantalum. Hydroxyapatite (HA) offers excellent biocompatibility but relatively poor mechanical properties, often requiring composite coatings with zirconium oxide and titanium oxide to enhance strength. Tantalum, on the other hand, possesses exceptional wear and corrosion resistance, with numerous successful clinical applications. Porous tantalum, often termed “metallic trabeculae,” is currently considered the most ideal material for joint surfaces.
Implantable medical devices used in surgery are high-end Class III medical devices and high-value medical consumables. Due to the stringent requirements for their specialized materials, demand for complex orthopedic components continues to grow significantly alongside advances in medical technology. The development of precision machining technology will continuously meet the demand for accurate and intelligent implantable components and specialized medical devices in orthopedic care, driving the healthy development of the medical device industry.
Conclusion
Orthopedic implants and surgical instruments belong to the category of high-end Class III medical devices and are considered high-value consumables. Due to stringent material requirements, the demand for complex orthopedic components continues to evolve alongside medical advancements.
The ongoing development of precision machining technologies will continue to meet the growing demand for accuracy and intelligence in orthopedic implants and specialized instruments, driving the sustainable growth of the medical device industry.